
RESEARCH
OVERVIEW
The Hirschi Lab is broadly interested in endothelial cell differentiation, proliferation, specification and function during development. Ongoing research is focused on understanding mechanisms of endothelial cell specification towards hemogenic, arterial, venous and lymphatic fates. The lab uses transgenic mouse models to study normal vascular development and to understand how regulatory processes are disrupted in developmental vascular malformations and diseases. The lab also uses human stem cell culture models to study the regulation of human endothelial cell differentiation and specification, and to study the functional roles of endothelial cells in regulating tissue-resident stem cells. For in vivo and in vitro studies, we employ next-generation sequencing and bioinformatic approaches to investigate underlying mechanisms. Insights gained from our cell and developmental studies are applied to collaborative projects focused on tissue engineering and regenerative medicine approaches.
CELL CYCLE REGULATION OF ENDOTHELIAL FATE
Vascular perfusion is required for all tissues in the body to provide nutrients and oxygen, remove waste products, maintain fluid homeostasis, and traffic immune cells. Functional blood and lymphatic vessels are lined with endothelial cells that must acquire specialized arterial, venous, capillary and lymphatic phenotypes to enable specialized functions [Marcelo, 2013; Marziano, 2021]. Endothelial cell specialization is associated with suppression of cell growth, but how signaling pathways that control endothelial cell cycle and fate are coordinated was not known. Recent studies in the Hirschi Lab have shown that shear flow forces induce a Notch-Cx37-p27 signaling axis that leads to cell cycle arrest, and this is required to enable arterial specification [Fang, 2017].
In subsequent studies using the Fluorescent Ubiquitination Cell Cycle Indicator (FUCCI) reporter, we found that endothelial cell cycle state determines propensity for arterial-venous fate. That is, endothelial cells in early G1 state are responsive to BMP signaling that promotes venous gene expression; whereas, endothelial cells in late G1 state are responsive to TGF-B signaling that promotes the expression of arterial genes [Chavkin, 2022]. Additionally, our studies using human embryonic stem cell-derived endothelial cells revealed that retinoic acid signaling promotes early G1 cell cycle state, which is required to enable hemogenic specification [Qiu, 2020]. Ongoing studies are further investigating underlying regulatory mechanisms, the role of endothelial cell cycle state in lymphatic specification, and the role of dysregulated of endothelial cell cycle state in vascular malformations and diseases.
Researchers: Gael Genet, Nick Chavkin, Corina Marziano, Shelby Cain, Jordon Aragon

HUVECs with FUCCI reporter
Selected Publications & Reviews:
-
Aragon JW, Nelson EA, Chavkin NW, Jackson MG, Heo W, Cain SR, Markowska M, Bradecamp GE, Genet G, Cwiek A, Hirschi KK. Emergence of endothelial subtypes and role of cell cycle control in arterial-venous specification during embryonic vascular development. Cell Reports. 2025
-
Acharya BR, Fang JS, Jeffery ED, Chavkin NW, Genet G, Vasavada H, Nelson EA, Sheynkman GM, Humphries MJ, Hirschi KK. Connexin 37 sequestering of activated-ERK in the cytoplasm promotes p27-mediated endothelial cell cycle arrest. Life Sci Alliance. 2023.
-
Chavkin NW, Genet G, Poulet M, Genet N, Marziano C, Vasavada H, Nelson EA, Kour A, McDonnell SP, Huba M, Walsh K, Hirschi KK. Endothelial Cell Cycle State Determines Propensity for Arterial-Venous Fate. Nature Comm. 2022.
-
Marziano C, Genet G, Hirschi KK. Vascular endothelial cell specification in health and disease. Angiogenesis. 2021.
-
Chavkin NW, Genet G, Poulet M, Genet N, Marziano C, Vasavada H, Nelson EA, Kour A, McDonnell SP, Huba M, Walsh K, Hirschi KK. Endothelial Cell Cycle State Determines Propensity for Arterial-Venous Fate. Nature Comm. 2022.
-
Marziano C, Genet G, Hirschi KK. Vascular endothelial cell specification in health and disease. Angiogenesis. 2021.
-
Qiu J, Nordling S, Vasavada HH, Butcher EC, Hirschi KK. Retinoic Acid Promotes Endothelial Cell Cycle Early G1 State to Enable Human Hemogenic Endothelial Cell Specification. Cell Rep. 2020.
-
Qiu J, Hirschi KK. Endothelial Cell Development and Its Application to Regenerative Medicine. Circ Res. 2019.
-
Hirschi KK, Dejana E. Resident Endothelial Progenitors Make Themselves at Home. Cell Stem Cell. 2018.
-
Fang JS, Coon BG, Gillis N, Chen Z, Qiu J, Chittenden TW, Burt JM, Schwartz MA, Hirschi KK. Shear-induced Notch-Cx37-p27 axis arrests endothelial cell cycle to enable arterial specification. Nat Commun. 2017.
-
Marcelo KL, Goldie LC, Hirschi KK. Regulation of endothelial cell differentiation and specification. Circ Res. 2013.
HEMOGENIC ENDOTHELIAL CELL BIOLOGY IN DEVELOPMENT

Hematopoietic stem and progenitor cells (HSPCs) that are essential for establishment and maintenance of the adult blood system are generated during embryonic definitive hematopoiesis. This process requires the specification of a subset of vascular endothelial cells to blood-forming, or hemogenic, endothelial cells that subsequently generate HSPCs via endothelial-to-hematopoietic transition [Wu, Annu Rev Physiol, 2021]. The mechanisms that regulate these processes are under intensive investigation, as their recapitulation in vitro from human pluripotent stem cells has the potential to generate autologous HSPCs for clinical applications [Qiu, 2019]. Research in the Hirschi Lab aims to uncover the developmental mechanisms of hemogenic endothelial cell specification and endothelial-to-hematopoietic transition, and apply insights gained to the generation of human HSPCs for clinical therapies.
Our studies, to date, revealed that hemogenic endothelial cell specification in the yolk sac and embryonic aorta-gonad-mesonephros (AGM) region is induced by retinoic acid signaling and a downstream signaling axis involving c-Kit, Notch and p27-mediated cell cycle control [Goldie, 2008; Marcelo, 2013]. Additionally, a recent collaborative study revealed a key role for miR-223 in zebrafish hemogenic specification via regulation of the endothelial cell N-glycome [Kasper, 2020]. Furthermore, the lab has generated protocols to isolate murine hemogenic endothelial cells [Fang, 2016] and to generate hemogenic endothelial cells from human pluripotent stem cells [Qiu, 2020; Nelson, 2021]. Ongoing studies employ these protocols, along with transgenic mouse models and next-generation sequencing approaches, to study hemogenic endothelial cell specification in the developing murine embryonic, AGM, yolk sac, and placenta, and to optimize the generation of human stem cell-derived HSPCs in vitro.
Researchers: Yinyu Wu, Liz Nelson, Ola Cwiek
Selected Publications & Reviews:
-
Wu Y, Paila U, Genet G, Hirschi KK. MicroRNA-223 limit murine hemogenic endothelial cell specification and myelopoiesis. Dev Cell. 2023
-
Nelson EA, Qiu J, Chavkin NW, Hirschi KK. Directed Differentiation of Hemogenic Endothelial Cells from Human Pluripotent Stem Cells. J Vis Exp. 2021.
-
Wu Y, Hirschi KK. Tissue-Resident Macrophage Development and Function. Front Cell Dev Biol. 2021.
-
Wu Y, Hirschi KK. Regulation of Hemogenic Endothelial Cell Development and Function. Annu Rev Physiol. 2021.
-
Kasper DM, Hintzen J, Wu Y, Ghersi JJ, Mandl HK, Salinas KE, Armero W, He Z, Sheng Y, Xie Y, Heindel DW, Park EJ, Sessa WC, Mahal LK, Lebrilla C, Hirschi KK, Nicoli S. The N-glycome regulates the endothelial-to-hematopoietic transition. Science. 2020.
-
Qiu J, Nordling S, Vasavada HH, Butcher EC, Hirschi KK. Retinoic Acid Promotes Endothelial Cell Cycle Early G1 State to Enable Human Hemogenic Endothelial Cell Specification. Cell Rep. 2020.
-
Qiu J, Hirschi KK. Endothelial Cell Development and Its Application to Regenerative Medicine. Circ Res. 2019.
-
Genet N, Bhatt N, Bourdieu A, Hirschi KK. Multifaceted Roles of Connexin 43 in Stem Cell Niches. Curr Stem Cell Rep. 2018.
-
Fang JS, Gritz EC, Marcelo KL, Hirschi KK. Isolation of Murine Embryonic Hemogenic Endothelial Cells. J Vis Exp. 2016.
-
Marcelo KL, Sills TM, Coskun S, Vasavada H, Sanglikar S, Goldie LC, Hirschi KK. Hemogenic endothelial cell specification requires c-Kit, Notch signaling, and p27-mediated cell-cycle control. Dev Cell. 2013.
-
Goldie LC, Lucitti JL, Dickinson ME, Hirschi KK. Cell signaling directing the formation and function of hemogenic endothelium during murine embryogenesis. Blood. 2008.
ENDOTHELIAL CELL REGULATION OF NEURAL STEM CELLS

To efficiently treat neurovascular diseases, there is an urgent need to promote parallel regeneration of both lost and/or damaged neurons and healthy revascularization, which are crucial for optimal tissue function. Neurovascular regeneration requires coordinated regulation of vascular endothelial cells that drive angiogenesis and neural stem cells (NSC) that drive neurogenesis [Goldberg, 2009]. In the adult murine brain, ongoing neurogenesis occurs in the subventricular zone (SVZ), which is the largest neural stem cell niche. It is well established that vascular endothelial cells are an integral feature of the SVZ and are capable of regulating NSC behavior via either paracrine or juxtacrine signaling [N Genet, 2021].
We found that gap junction protein connexin (Cx)43, is highly expressed by both endothelial cells and NSC [Goldberg, 2013] in the SVZ. Ongoing studies aim to understand the role of Cx43-mediated interactions between NSC and endothelial cells in the regulation of SVZ NSC behavior and neurogenesis [N Genet, 2018]. For in vivo studies, we use mouse models (Cx43Cdh5CRE-iERT2 and Cx43GlastCRE-iERT2) wherein Cx43 can be inducibly deleted in either endothelial cells or NSCs, respectively. We further explore regulatory mechanisms in vitro using a transwell co-culture system of NSC (ANS4-GFP) and brain microvascular endothelial cells (b.END3). In addition, in collaborative studies, we have shown that encapsulating ANS4-GFP with b.END3 in polyethylene glycol (PEG)-based microbeads allows maintenance of NSC quiescence and accelerated delivery into the adult brain post-implantation [Matta, 2019]. Gaining further insights into the mechanisms by which endothelial cells regulate NSCs will facilitate the optimization of injectable biomimetic niches that maintain NSC survival, migration, and differentiation post-injection for the treatment of acute brain injury and progressive degeneration.
Researcher: Nafiisha Genet
Selected Publications & Reviews:
-
Genet N, Genet G, Chavkin NW, Paila U, Fang JS, Vasavada H, Goldberg JS, Acharya BR, Bhatt N, Baker K, McDonnell SP, Huba M, Sankaranarayanan D, Ma GZ, Eichmann A, Thomas JL, ffrench-Constant C, Hirschi KK. Connexin 43-mediated neurovascular interactions regulate neurogenesis in the adult brain subventricular zone. Cell Reports 2023.
-
Genet N, Hirschi KK. Understanding neural stem cell regulation in vivo and applying the insights to cell therapy for strokes. Regen Med. 2021.
-
Matta R, Lee S, Genet N, Hirschi KK, Thomas JL, Gonzalez AL. Minimally Invasive Delivery of Microbeads with Encapsulated, Viable and Quiescent Neural Stem Cells to the Adult Subventricular Zone. Sci Rep. 2019.
-
Genet N, Bhatt N, Bourdieu A, Hirschi KK. Multifaceted Roles of Connexin 43 in Stem Cell Niches. Curr Stem Cell Rep. 2018.
-
Coşkun S, Chao H, Vasavada H, Heydari K, Gonzales N, Zhou X, de Crombrugghe B, Hirschi KK. Development of the fetal bone marrow niche and regulation of HSC quiescence and homing ability by emerging osteolineage cells. Cell Rep. 2014.
-
Goldberg JS, Vadakkan TJ, Hirschi KK, Dickinson ME. A computational approach to detect gap junction plaques and associate them with cells in fluorescent images. J Histochem Cytochem. 2013.
-
Goldberg JS, Hirschi KK. Diverse roles of the vasculature within the neural stem cell niche. Regen Med. 2009.
REGULATION OF LYMPHATIC ENDOTHELIAL CELL SPECIFICATION

The lymphatic circulation is a specialized network of vessels responsible for maintaining tissue fluid homeostasis, immune cell transport and lipid absorption. Establishment of a functional lymphatic circulation is essential for normal embryonic development. The lymphatic circulation develops shortly after the formation of the blood vasculature in a series of steps including lymphatic endothelial cell (LEC) specification, migration, maturation, and valve formation. During early development, LECs are specified from Lyve1-expressing lymphatic progenitor cells in the cardinal vein. Once committed to a lymphatic fate, Prox1-expressing LECs migrate out of the cardinal vein to form the primitive lymphatic plexus, which undergoes remodeling into a mature lymphatic vasculature containing vessels with specialized intraluminal valves. Disruption in any of these developmental processes contributes to the pathogenesis of congenital lymphedemas, as well as postnatal obesity, atherosclerosis, and neurological disorders. It is essential to understand the underlying mechanisms regulating lymphatic progenitor development and LEC specification in order to identify novel therapeutic targets that may restore a functional lymphatic network in patients affected by lymphedema and other associated disorders. Currently we are interested in the role of retinoic acid (RA), and downstream signaling effectors, that promote the development of lymphatic progenitors from venous endothelial cells in the cardinal vein of the developing embryo. Other projects investigate the mechanism by which LECs are specified from progenitors and how a subset responds to changes in shear stress to form functional intraluminal valves. In all processes, we are interested in understanding how cell cycle state may contribute to the integration and response to developmental cues and how this affects the specification and development of a functional lymphatic vascular system.
Researchers: Corina Marziano, Ola Cwiek, Danya Sankaranarayanan, Maddie Jackson, Valentin Delobel
Selected Publications & Reviews:
-
Marziano C, Genet G, Hirschi KK. Vascular endothelial cell specification in health and disease. Angiogenesis. 2021.
ENDOTHELIAL CELL CYCLE CONTROL IN VASCULAR MALFORMATIONS

Blood vessels are highly organized networks of arteries, capillaries, and veins. In patients with mutations that disrupt the mechanisms that regulate blood vessel formation – as in the genetic disease Hereditary Hemorrhagic Telangiectasia (HHT) – this can lead to the formation of abnormal, disorganized blood vessels (termed vascular malformations) that are prone to sudden and serious bleeding.
HHT affects 1 in 5,000 live births. Because it can vary in its clinical presentation, HHT can be difficult to diagnose. Symptoms often begin in childhood and progress in severity, but patients typically do not receive a definitive HHT diagnosis until >40 years of age. Drugs currently being used to treat this disease are helpful to many individuals, but do not work for all patients and cause many deleterious ious side effects. Therefore, new drug treatments are needed to help people afflicted with this illness.
Our work shows that proper endothelial cell cycle control is important for normal blood vessel formation. Using new mouse models to study changes in endothelial cell cycle in blood vessels in normal and disease conditions, we found that uncontrolled cell cycle progression leads to vascular malformations. We are investigating the effectiveness of FDA-approved cell cycle modulating drugs for the prevention and/or regression of vascular malformations. Thus, our central hypothesis is that dysregulated endothelial cell cycle control is a key factor leading to vascular malformations and that drugs that regulate endothelial cell cycle could represent a new therapeutic strategy for HHT patients.
Researcher: Gael Genet
Selected Publications & Reviews:
-
Genet G, Genet N, Paila U, Cain SR, Cwiek A, Chavkin NW, Serbulea V, Figueras A, Cerda P, McDonnell SP, Sankaranarayanan D, Huba M, Nelson EA, Riera-Mestre A, Hirschi KK. Induced endothelial cell cycle arrest prevents arteriovenous malformations in hereditary hemorrhagic telangiectasia. Circulation. 2024
-
Marziano C, Genet G, Hirschi KK. Vascular endothelial cell specification in health and disease. Angiogenesis. 2021.
DEVELOPMENTAL GENOMICS
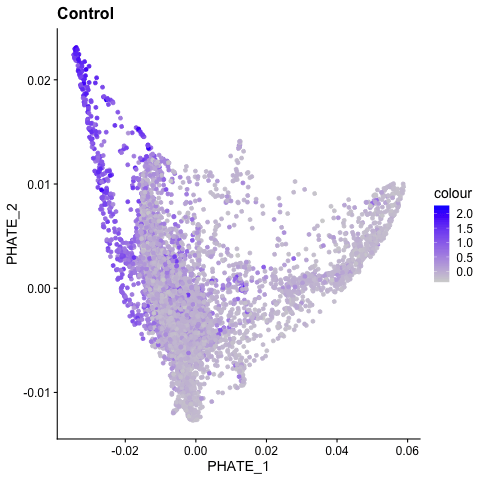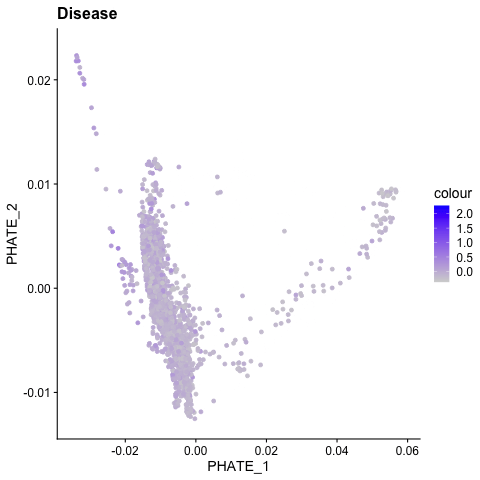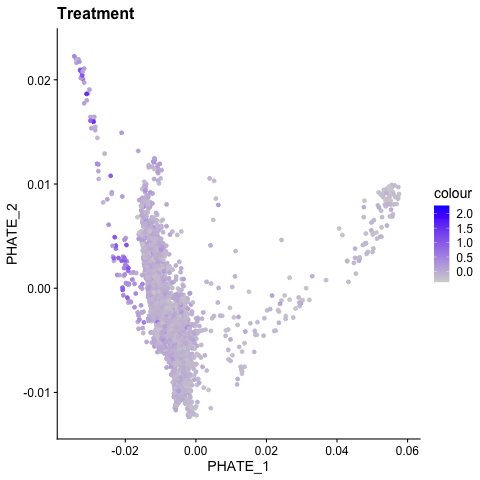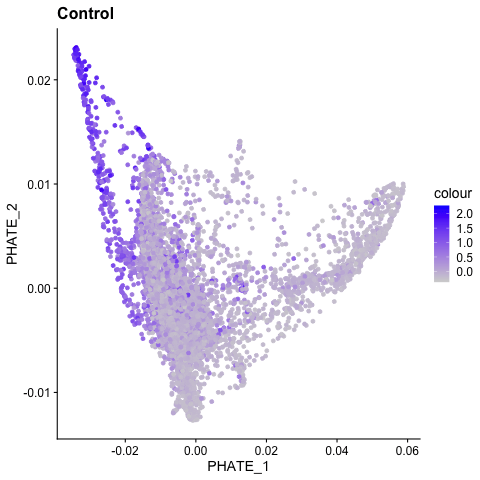
BIOINFORMATIC & SYSTEMS BIOLOGY APPROACHES
Recent advances in bioinformatic approaches and their application to vascular biology have revealed novel mechanisms of endothelial cell differentiation and regulation. For example, single cell RNA sequencing has revealed the broad heterogeneity of endothelial cell phenotypes in developing vascular beds, adult tissues, and in multiple disease states [Chavkin, 2020]. In ongoing studies, the Hirschi lab combines bulk and single cell analyses with systems biology approaches to discover novel mechanisms of endothelial cell differentiation, specification and function. For such studies, the lab has recently generated a protocol for the isolation of retinal endothelial cells that is compatible with next-generation sequencing applications [Chavkin, 2021; Chavkin, 2021].
GENETIC ANALYSIS OF HUMAN VASCULAR DEFECTS
Birth defects affect 1 in every 33 babies born in the United States each year, affecting ~120,000 babies every year. And each year, hospital costs for US children and adults with birth defects exceed $2.6 billion, not including outpatient care. Congenital defects of the cardiovascular system account for almost half of all birth defects in the US and beyond, and ~40% are due to gene mutations that are either inherited or arise during development.
The Hirschi lab aims to define the gene mutations that cause these defects so that better prevention, diagnostic and treatment strategies can be developed. They are specifically working to uncover the genes and cell types that cause vascular malformation and dysfunction. However, as an integral component of the UVA Developmental Genomics Center, they more broadly aim to bridge developmental biologists from multiple clinical and basic science departments at UVA, and with genomic and translational scientists at the Genomics and Bioinformatics Research Institute (GBRI) at Inova. A major goal is to translate developmentally related databases into disease gene discovery and therapeutic targeting.
Researchers: Uma Paila, Jordon Aragon, Gael Genet
Selected Publications & Reviews:
-
Chavkin NW, Cain S, Walsh K, Hirschi KK. Isolation of Murine Retinal Endothelial Cells for Next-Generation Sequencing. J Vis Exp. 2021.
-
Chavkin NW, Walsh K, Hirschi KK. Isolation of Highly Purified and Viable Retinal Endothelial Cells. J Vasc Res. 2021.
-
Chavkin NW, Hirschi KK. Single Cell Analysis in Vascular Biology. Front Cardiovasc Med. 2020.